In a conference in D.C. this week my mentor, Dr. Deheyn, is presenting some of the work I've done in creating an experiment that will test the effects of a magnetic field on the bioluminescent mucus of the tube worm Chaetopterus variopedatus. Since the mucus is Ferrous and a reaction involving Fe+2 and H2O2 (peroxide) is necessary for the organism to luminesce, there is a possibility that magnetism might increase the luminosity (brightness) of the mucus by somehow stimulating the ferrous reaction within the mucus. Previous tests were conducted with rare earth magnets (see this blog for the previous test procedures), but after I obtained erratic results I decided to construct an electromagnet (click here for construction process), which would produce a uniform electromagnetic field that could be regulated.
PROTOCOLS
An electromagnet was created by coiling 30-Gauge wrapped wire around a plastic tube multiple times (more coils = stronger magnetism) designed to hold a 1.5 ml tube. This electromagnet was connected to a Laboratory DC Power Supply and supplied with 17.35 Volts of coarse current. For the experiments, two 1.5 ml tubes containing 750 µl of Artificial Sea Water and 750 µl of Raw C. variopedatus mucus apiece were prepared.
One C. variopedatus worm was extracted from its tube and brought into the lab, where it was subsequently decapitated and soaked in 1000 µl of Potassium Chloride for 7 minutes and 30 seconds. Then the extracted mucus/KCl solution was diluted with 750 µl ASW. 100 µl samples were taken from each tube and analyzed for Kinetics on a Luminometer.
TEST 1
The samples from Test 1 were analyzed in 5 minute intervals for 50 minutes and showed no significant trends over the 10 analyzed samples.
TEST 2
Test 2 was conducted with the same protocols. The altered variable was the interval at which samples were taken. 100 µl samples were pipetted in 10 minute intervals and analyzed rather than 5 minute intervals. The data showed no significant trends in Test 2 either, however.
TEST 3
The very nature of this bioluminescent mucus (of any mucus, in fact) is that it is very viscous and adhesive. Therefore, one reason why the results were so erratic could have been due to the fact that the samples were from the same batch, but there was no guarantee that the samples had contained similar proportions of mucus to ASW. Testing one sample of mucus and then testing a totally different sample to observe the luminescent decay would not necessarily demonstrate the decay of the first sample. So for Test 3, no samples were taken at intervals.
Instead, two Luminometer analysis tubes were filled with750 µl of the raw mucus/KCl mixture apiece. One was placed in the control container and the other in the electromagnet. The whole 750 µl solution was placed into the luminometer at 10 minute intervals. The solution remained undisturbed by pipets, as no samples were extracted. The ability to place the whole tube inside the luminometer allowed for a consistent luminous decay to appear on the graph since it was the same (not different) sample being tested each time. In addition, the accuracy of the reading on the mucus within the magnetic field was equivalent to the accuracy of an extracted solution. In other words, if there were any bad effects from taking the sample away from the magnetic field, that discrepancy was consistent throughout all tests as far as data collection goes, however, if the mucus required constantsubjection then the 10 seconds for which it was outside of the field might have significantly affected the experiment.
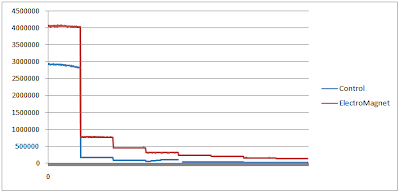
The 3rd column shows how much the luminosity has decreased during the ten minute intervals. Surprisingly enough, the Total Decay, which was the difference between the luminosity after 70 minutes and the initial luminosity, was greater in the mucus in the electromagnet than in the control. The mucus in the electromagnet initially started out much higher, but subsequently dropped to hover above the luminosity of the control. Although in the end it was still brighter, it decayed much more. If anything, it stimulated higher initial luminosity which possibly led to more decay afterwards. Nonetheless, the Control had a quicker rate of decay than the Electromagnet.
So far, the best method seems to call for 750 µl of raw mucus/KCl solution in luminometer tubes, analyzed for luminosity every five minutes and testing the effects of adding metal or any other modifications in the apparatus. The length of the test would have to be determined by the point at which the luminescent activity is no longer significant. Test 3 was stopped before the luminescent activity dropped to that level.
The chart below displays the differences in average luminosity throughout the test and the rate of decay in order to determine which one decayed more and which one decayed faster.
Since the Control decayed at a faster rate than the mucus subjected to an Electromagnetic field, I'm going to continue using the protocols of Test 3 for more experiments to make sure that this trend is not anomalous.